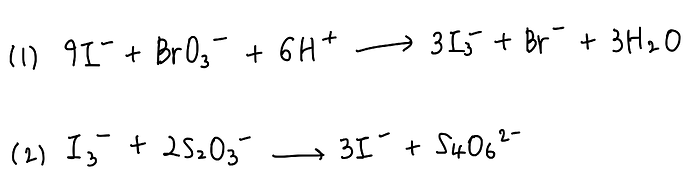To an acidic aqueous solution of KBrO3 (100mL, 0.1 mol/L) is added 100mL of an aqueous solution containing 0.006 moles of K2S2O3 and 0.09 moles of KI.
The following quantitative reactions occur:
Reaction (2) is instaneous, while reaction (1) is relatively slow.
By the time all the S2O3 2- anion present in solution has reacted, how many moles of BrO3- will have been consumed?
solution: BrO3- : I3- = 1:3
I3- : S2O3- = 1:2
–>BrO3- : I3-:S2O3 = 1:3:6
BUT I don’t know why I can use ratio…