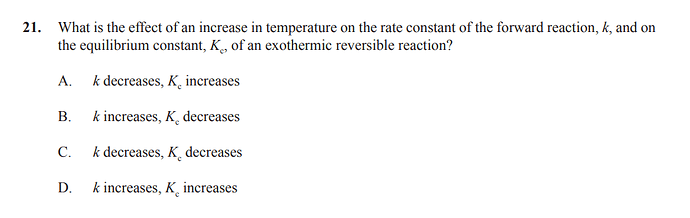Hi Ari,
In our last meeting we solved IB chemistry 2012 past paper together, here are our questions:
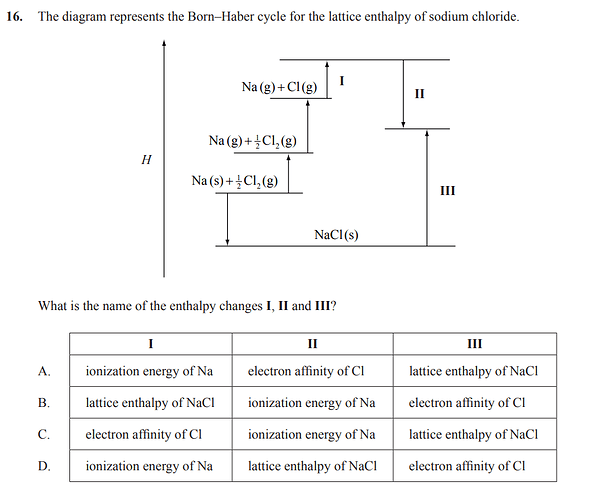
The answer is A).
We could not figure out what enthalphy changes I) and II) were.
The answer is B).
Is our reasoning correct for the change of k:
K increases since there is more energy added to the reaction, both the forward and reverse rate increase.
(I got confused I thought the k would decrease since the reaction is exothermic so the equilibrium would shift to the reverse reaction. So the rate increases but the concentration of reactants decreases?)
Answer is B)
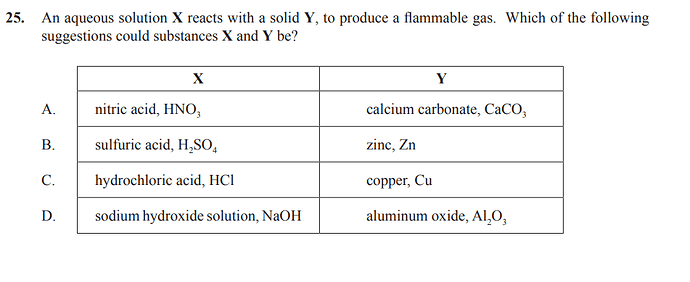
We could not determine which are solids, and which reactions would produce a flammable gas.
Thank you for your help!