Hey!
A great question ![]()
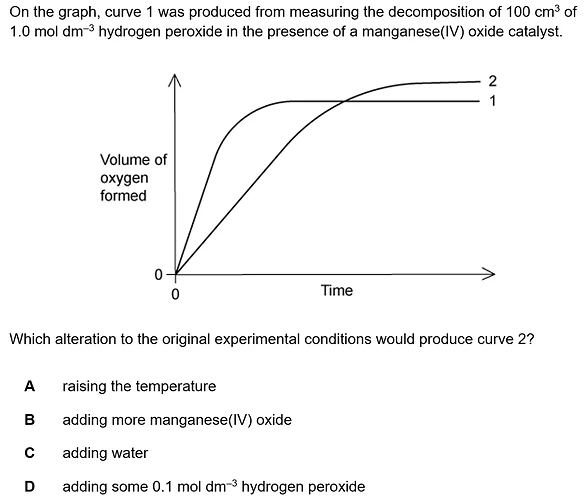
We see graph number 1 is steeper than graph number 2, and that the final amount of oxygen is higher at the end of the process.
If we would add water to the reaction, it would not change the rate nor the ultimate total amount of the oxygen produced, so it is false.
Heating up the process would make graph number two even steeper relative to graph number 1, as the reaction would be more rapid. we can see this is not the case.
Adding manganese will speed up the process too, but would not affect the total amount of oxygen produced. this is also true for raising the temperature.
Now after eliminating the other options lets look at option D. Adding more reactants would make the total process slower, as we have some “free” reactants that are not bound to the catalyst. the relative process would be slower simply because the catalyst- reactants ratio decreases. This would also yield a higher amount of product, which is indeed the case in graph number 2.
Hope is clarify things