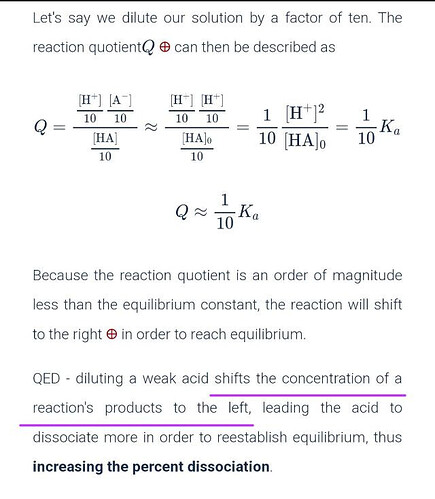
Just a last question: if the reaction first shifts to the left, it produces more acid so it must be so that the acid will react with added water in the following step to replace the initial ions and create more ions. By doing so the equilibrium can be reestablished. Am I right?
Answer credited to @Asafmen
It does not produce more acid, it momentarily decreases the ion concentration and the behaviour of the acid- relative to the new ratio. You give the acid more ground to work on.