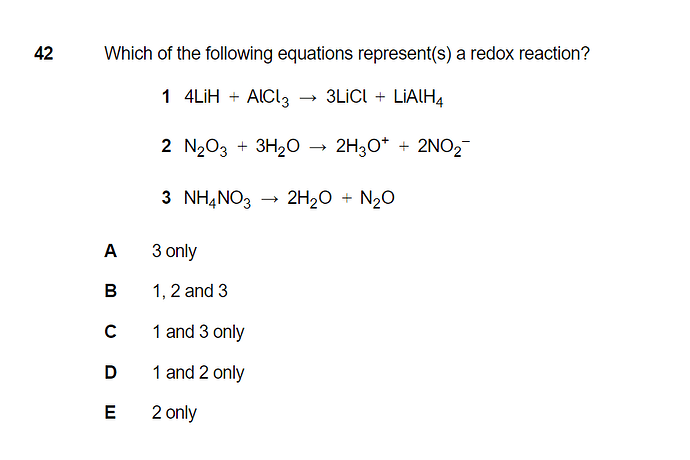
To define a redox reaction we should look for any e transfer, right? Mb some changes in oxidation states of elements. I’m struggling with defining oxidation states of elements in those substances for example nh4n03 how to do this? Mb somebody could showme on paper
Well, this question is a bit complicated. Firstly, as we know, elements in a compound must reach a stable state with their outermost electron number to be 8,2 or 0. Then we can simply use the atomic number of an element to deduce whether it loses or gains electrons. Finally, the number of electrons they gain or lose determines the exact valence.
To determine whether an element gains or loses electrons involves chemical bond and electronegativity. They are too complicated to be illustrated here, you’d better check your text book.
I know how to do it with simple compounds, but these are a bit different I I guess. Thank you anyway!!
Thank you, I understood ![]()
Oxidation numb of Hydrogen is -1 with metal for option 1.
For option 3, NH4NO3 should be thought first as NH4+, NO3- and then you can figure out oxi numb there.
After that you can compound them.