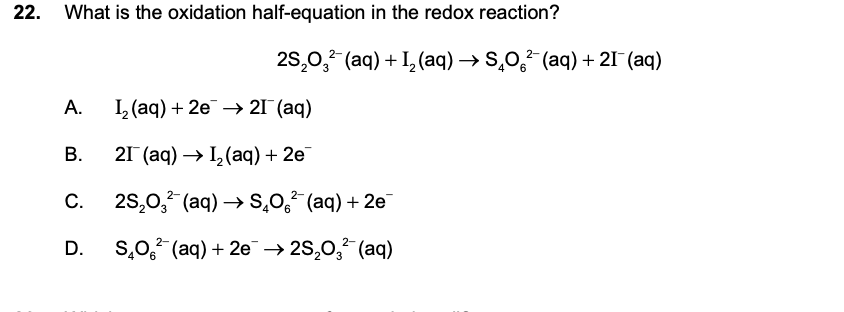A is wrong because Iodine is getting reduced which means it is gaining 2 electrons.
Similarly the second equation is wrong as then have wrote the product in place of the reactants.
** Moreover they asked for the oxidation half and Iodine comprises the reduction half as its oxidation number changes from 0 to -1.
C. is correct because it accurately demonstrates the movement of electrons S2O3 is being oxidised as the oxidation number is changing from +2 to +2.5.
And D is wrong as the electrons are lost so they should be on the product side and this wrongly demostrates the movement of electrons.