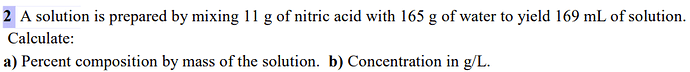hi could you include the correct solution?
Yeah sure
It is a) 6.25 % b) 65.09 g/L
A) mass % composition = m HNO3 / total m * 100
total mass=11+165=176g
=11/176 * 100=6,25%
B) C=n/V with n(HNO3)=m/Mw=11/73
C=11/73 / 0,169L = 0,891mol/L
m=n * Mw so C = 0,891 * Mw = concentration in g/L
0,0891 * 73 = 65,09 g/L
1 Like
hey juliette, i did not understand your explanation, could you please explain again?
it does, thank you so much
1 Like