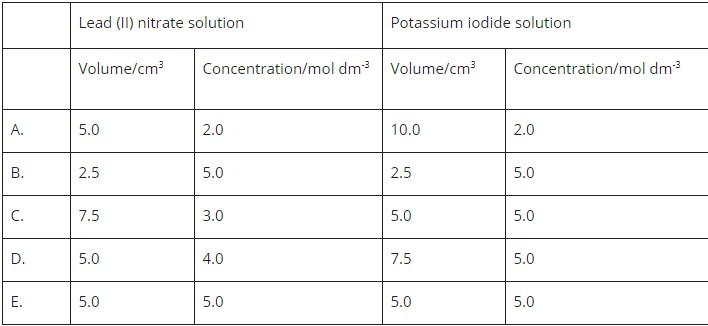
The reaction between lead (II) nitrate solution and potassium iodide is shown below. When the two solutions are mixed in a test tube, the height of the lead (II) iodide precipitate formed can be measured. Which combination of solutions would produce the greatest height of the precipitate?
The answer is D. May i know the calculation?
1 Like
Hi! I’ll tell you the steps so you can calculate it!
First you write the equation of the reaction and balance it. Then for each option find the moles using concentration x volume(you can start from the bottom since it has the highest concentration - would give you more moles and saves you time). From there you can identify the limiting reactant and the mole ratio to see how many moles of the product you get. Greatest precipitate means the most moles of product.
You can kinda see by just looking at the table to figure out the answer. For example: both D and E have the higher value of concentrations for lead (II) nitrate. However, you can see in D, it has a higher volume of potassium iodide solution which would give more moles. And therefore is the most possible answer.
Let me know if you need the calculation done step by step!
4 Likes
hey! I was thinking instead of writing the whole equation can I calculate it with the net ionic equation as it is just a precipitate reaction, do you think that would work?
2 Likes
Yep that’s fine too I just write the whole equation cause it’s just easier to balance.
2 Likes