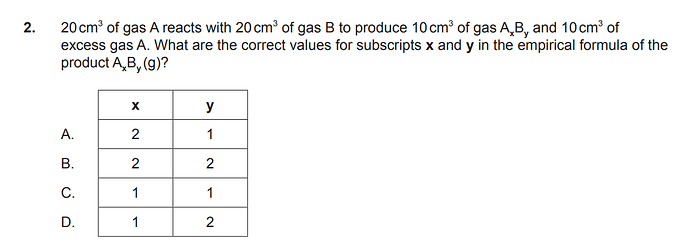
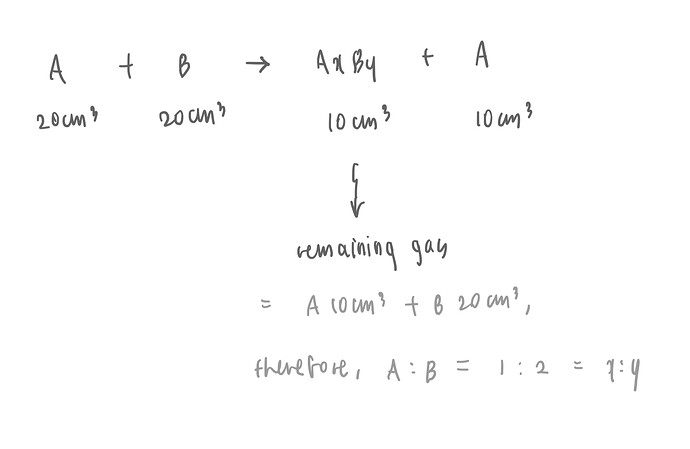
I think it is supposed to be easy and simple, but I still couldn’t approach the question. How to solve this question? The answer is D.
I still cant understand much. I prefer an answer with a reason to understand. Thank you!
Hi, so if we had 20cm3 of A and 20cm3 of B in the reactants,
we will have 20cm3 of A and 20cm3 of B in the products.
In case of products (AxBy + A), since excess gas A is 10cm3, AxBy will be addition of A 10cm3 (remaining) and B 20cm3.
Therefore x = 1, y = 2.
1 Like