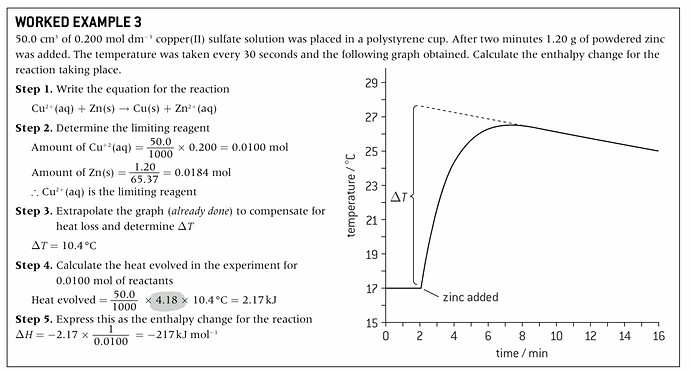
I understand how it’s worked in this question, but I’m not sure why it’s
Heat evolved = mass of copper sulfate soln * specific heat of water * change of temperature,
(Highlighted part in step 4)
when water is not involved in any of the processes.
Thanks!
Hello!
I hate chemistry and specially energetics so i never imagined to answer a chem question one day hahahah, but anyways:
We always take the specific heat capacity of water which is always 4.18. Unless stated otherwise (if another specific heat capacity was mentioned in the question basically) then you will use it.
This is because there is much more water in the solution since it is the solvent so the specific heat capacity of anything else is neglected (altho it isnt accurate which is why i dont like the whole chapter it is very uncertain while it could have been more accurate but oh well)
Hope this helps, if anyone else has another, more accurate answer, please share!!
1 Like