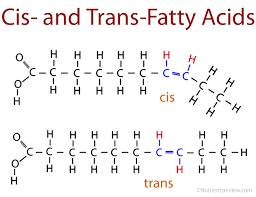
I think that cis have higher melting point than trans bc of their high dipole moment, stronger intramolecular forces.
The boiling point of a substance depends on the strength of the intermolecular forces. In the case of cis and trans unsaturated fats, the structure of the molecules affects these forces.
In general, trans fats have a higher melting point than cis fats. Trans fats are more symmetrical and can pack together more tightly, creating stronger intermolecular forces. The cis configuration introduces a bend in the molecule that prevents tight packing, leading to weaker intermolecular forces.
However, it’s important to note that for the IMAT exam and generally, the boiling points of fats are rarely discussed because fats undergo decomposition or oxidation before they reach a temperature where they would boil. The comparison is usually made with melting points, which are easier to measure and more relevant in practical applications.